Perfluoroalkanes in AMBER-ii Force Field
|
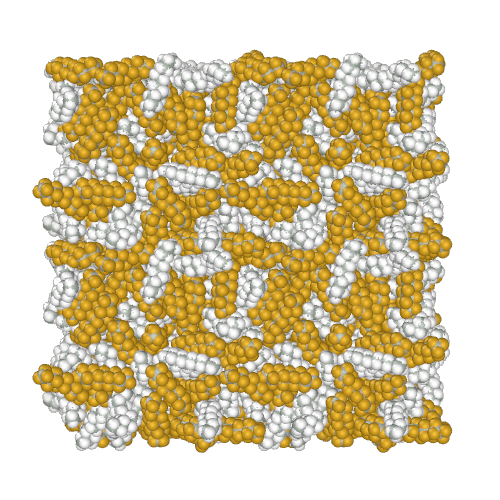
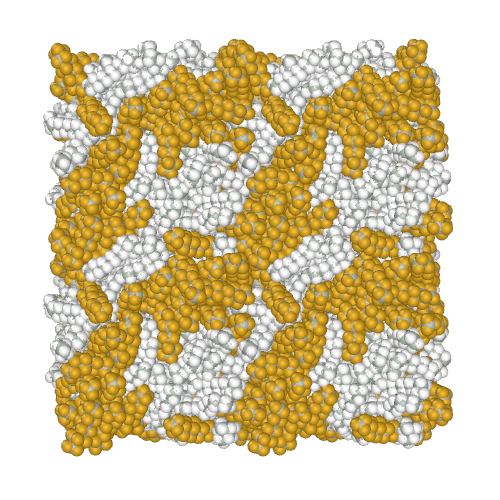
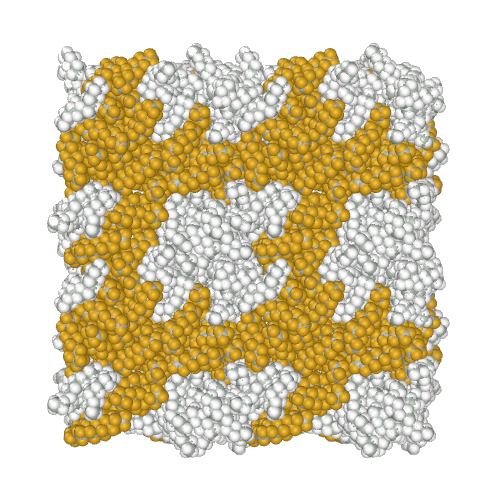
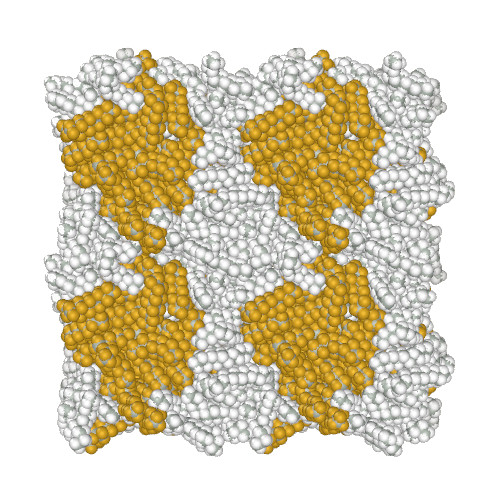
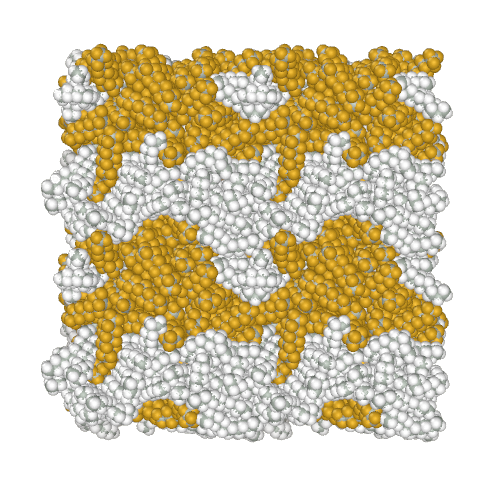
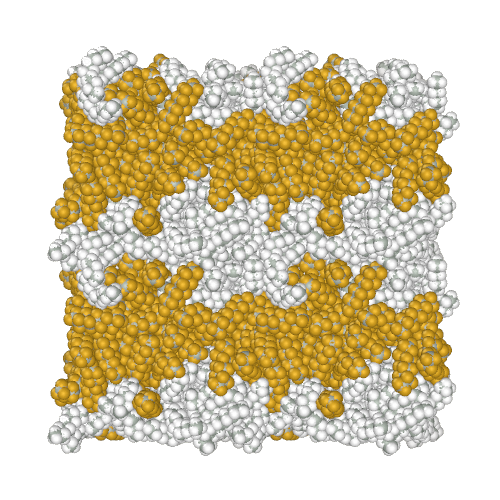
Perfluoroalkanes are famous for their properties similar to normal alkanes, but with interesting differences that determine their practical application. One of the practically useful but difficult to model properties is the mutual insolubility of higher liquid alkanes and perfluoroalkanes. Because of assigning a relatively high partial charge of −0.37e to fluorine atoms, the AMBER-ii reproduces the mutual insolubility1. Simulation of phase separation in a mixture of C12H26 and C9F20
It is interesting to note that the obtained parameters provide the conformational properties of linear perfluoroalkanes without the introduction of torsion potentials1.
1 Alexei M. Nikitin, Yury V. Milchevskiy and Alexander P. Lyubartsev AMBER-ii: New Combining Rules and Force Field for Perfluoroalkanes. J. Phys. Chem. B, 2015, 119 (46), pp 14563–14573 DOI 10.1021/acs.jpcb.5b07233
|
 Copyright © 2006-2011 Agile Molecule. All rights reserved
Copyright © 2006-2011 Agile Molecule. All rights reserved